Posted by Chrom Tech on 16th Oct 2025
Screening for Vitamin E Acetate in Cannabinoid Potency Workflow
The rise in e-cigarette and vaping product use has brought significant public health concerns. The United States is currently facing an outbreak of lung injuries known as EVALI (E-cigarette, or Vaping, product use Associated Lung Injury). As of November, more than 2,000 EVALI cases have been confirmed, resulting in over 40 deaths, according to the CDC.
Vitamin E Acetate: A Chemical of Concern
The Centers for Disease Control and Prevention (CDC) identified vitamin E acetate as a key compound of concern in EVALI investigations. This vitamin E-derived oil has been detected in every fluid sample collected from the lungs of affected patients. It is sometimes used illicitly as a thickening or diluting agent in THC vaping liquids to enhance appearance and profitability.
Although vitamin E is considered safe for topical or oral use at low concentrations, it can become harmful when inhaled. Studies suggest that inhaling this compound may compromise lung function and contribute to chemical-induced lung injury. This discovery has prompted cannabis testing laboratories to add vitamin E acetate screening into their potency and safety analysis workflows.
Expanding the Cannabinoid Potency Workflow
Most states that have legalized cannabis require analytical testing for potency, pesticides, mycotoxins, and residual solvents. However, due to the recent identification of vitamin E acetate as a harmful additive, it is not yet included in many state regulatory testing requirements. Despite this, many laboratories are proactively incorporating screening methods to safeguard consumer health and maintain product integrity.
To support this initiative, Restek has revised its cannabinoid analysis workflow to include vitamin E acetate within a modified potency assay. The compound can be effectively separated and quantified using the Restek Raptor ARC-18 column, which provides excellent selectivity and stability for complex cannabinoid matrices. Restek continues to explore additional methods for rapid screening as research on this compound expands.
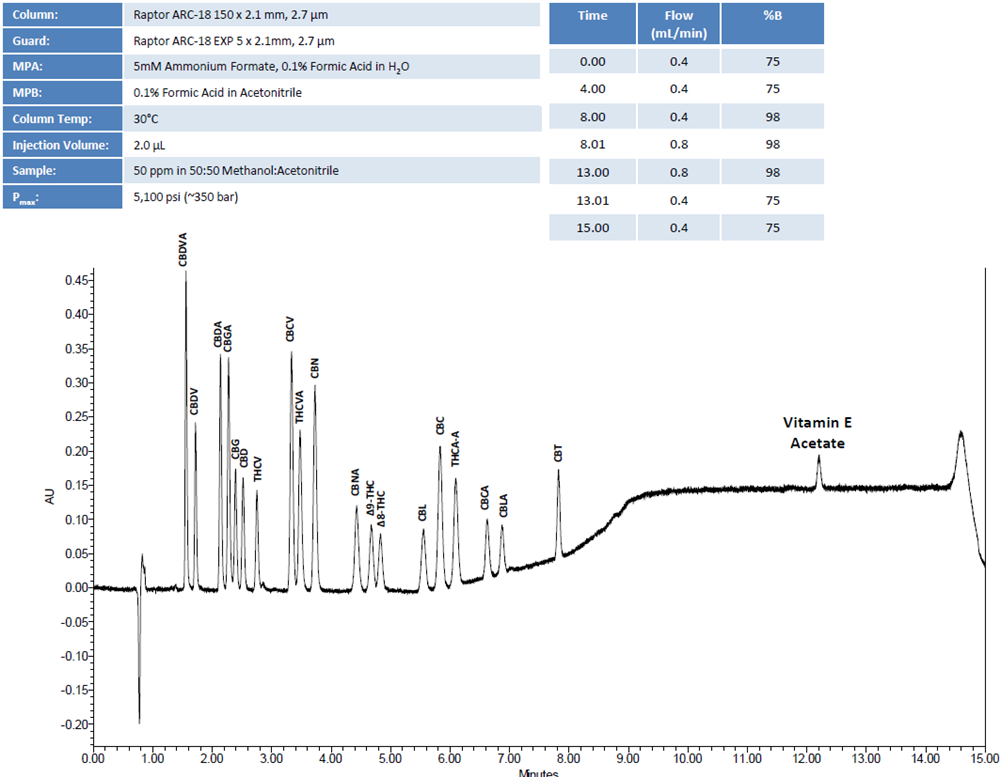
Method Considerations for Vitamin E Acetate Detection
Screening workflows for vitamin E acetate typically employ liquid chromatography (LC) coupled with mass spectrometry (MS)</strong) to ensure high sensitivity and specificity. The addition of this compound to existing cannabinoid workflows requires careful optimization of sample preparation, mobile phase composition, and column selectivity to maintain accurate potency measurements.
*While current evidence strongly suggests a link between vitamin E acetate and EVALI, other substances may also contribute to these injuries. Ongoing research continues to identify and assess additional chemicals of concern within vaping products.
Partner with Chrom Tech
Chrom Tech is a Minnesota-based worldwide distributor of chromatography and mass spectrometry supplies. We provide vials, well plates, syringe filters, solvent waste containers, and other essential chromatography consumables. Contact Chrom Tech to learn more about tools that support cannabinoid potency and safety testing workflows.
Frequently Asked Questions
Why is Vitamin E Acetate dangerous when inhaled?
Vitamin E acetate can interfere with normal lung function when vaporized and inhaled, potentially leading to chemical pneumonitis and lung injury. While safe for topical or dietary use, it poses risks when inhaled in aerosolized form.
How can labs test for Vitamin E Acetate in cannabis products?
Labs can integrate Vitamin E acetate screening into existing potency workflows using LC/MS or GC/MS methods. The Restek Raptor ARC-18 column provides high-resolution separation suitable for detecting Vitamin E acetate in complex matrices.
Is Vitamin E Acetate currently regulated in cannabis testing?
As of now, most state cannabis testing regulations do not mandate Vitamin E acetate analysis. However, many labs include it voluntarily to ensure consumer safety and product transparency.

