Posted by Chrom Tech on 28th Oct 2025
What Causes Peak Tailing in HPLC?
By Chrom Tech – Your Chromatography Consumables Partner Since 1983
Understanding Peak Tailing in HPLC
In liquid chromatography, a perfect chromatographic peak is symmetrical—known as a Gaussian peak. Symmetry and narrow width are essential for accurate quantification and resolution. However, poor peak shapes such as peak tailing or fronting can compromise analytical accuracy and reproducibility. Understanding the root causes of peak tailing in HPLC is critical for method optimization and maintaining high data quality.
How Peak Shape is Measured
USP Tailing Factor
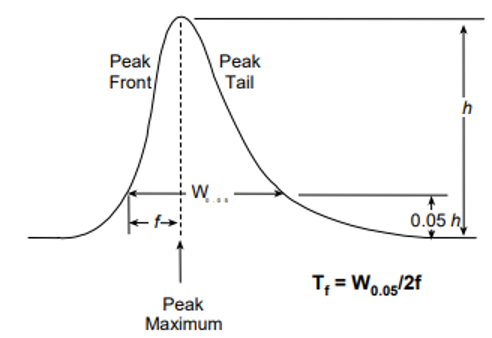
- W0.05:
- Width of the peak at 5% of its height.
- f:
- Distance between the peak maximum and the leading edge at W0.05.
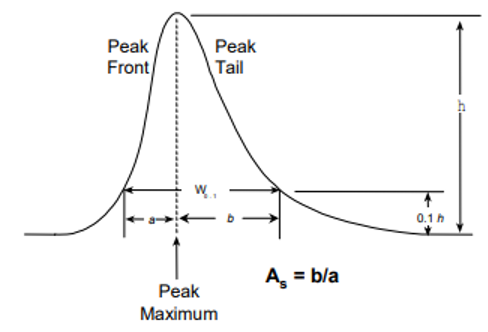
Asymmetry
Peak Width at Half Height
Primary Causes of Peak Tailing in HPLC
- Silanol Interactions: Ionized silanols on silica-based columns interact with basic analytes, especially at mid-pH levels, causing tailing.
- pH Effects: When mobile phase pH approaches the analyte’s pKa, uneven ionization leads to asymmetrical peaks.
To minimize these effects, use endcapped or polar-embedded HPLC columns. Endcapped phases block residual silanol activity, while polar-embedded phases provide additional shielding for basic compounds.
- Mobile Phase & Buffer: Avoid operating near the analyte’s pKa. Buffering stabilizes pH and improves peak symmetry. The choice of organic modifier (methanol vs. acetonitrile) also influences peak shape.
- Extra-Column Effects: Long or wide tubing increases dispersion and tailing. Use narrow internal diameter (0.005") PEEK tubing to minimize dead volume.
Why Peak Tailing Matters
Peak tailing is not merely aesthetic—it indicates system inefficiency or chemical interaction issues that affect quantitative accuracy and reproducibility.
Impact on Data Accuracy
- Obscures nearby analytes, leading to integration errors.
- Complicates quantification and identification of co-eluting compounds.
How to Correct Peak Tailing
- Adjust Detection Wavelength: Reduces interference from co-eluting impurities.
- Enhance Column Efficiency: Use smaller particle sizes or longer columns for improved symmetry.
- Use High-Efficiency Columns: Select advanced bonded-phase columns from brands like Agilent or Restek for reduced silanol activity.
Peak Tailing in Reversed-Phase HPLC
In reversed-phase systems, tailing arises from both hydrophobic and polar secondary interactions, particularly between basic analytes and deprotonated silanol groups (pH > 3.0). These interactions distort peak symmetry and reduce reproducibility.
Operating at High pH for Basic Compounds
Elevating pH suppresses acid ionization, improving separation for basic analytes. Modern HPLC columns with bi- or tridentate bonding provide stability against silica hydrolysis, maintaining consistent performance under alkaline conditions.
Role of Sample Clean-Up
Effective sample clean-up is essential to prevent tailing and contamination. Techniques like Solid Phase Extraction (SPE) eliminate interfering compounds that degrade column performance and affect quantitation.
Benefits of Proper Sample Clean-Up
- Removes interferences from complex matrices.
- Improves peak symmetry and analytical precision.
- Enhances sensitivity and reproducibility.
- Extends column life by minimizing contamination.
Conclusion
Peak tailing in HPLC can distort results and reduce analytical confidence. By understanding and controlling factors such as silanol interactions, mobile phase chemistry, and tubing design, laboratories can restore symmetry and accuracy. Consistent use of high-quality columns and proper sample clean-up ensures sharper, more reproducible peaks—supported by Chrom Tech’s reliable chromatography supplies.
Frequently Asked Questions
What is peak tailing in HPLC?
Peak tailing describes an asymmetric chromatographic peak with a stretched trailing edge, often caused by interactions between analytes and the stationary phase.
What causes peak tailing?
Common causes include silanol interactions, pH near analyte pKa, extra-column volume, and impurities from unfiltered samples.
How can I reduce peak tailing?
Use endcapped HPLC columns, maintain optimal pH buffers, minimize dead volume, and ensure clean sample preparation.
Why is peak tailing problematic?
Tailing reduces resolution, obscures adjacent peaks, and can cause inaccurate quantification and peak integration.
Does pH influence peak tailing?
Yes. Operating near an analyte’s pKa can increase silanol interactions, while adjusting pH can improve symmetry and retention consistency.
What is an acceptable USP tailing factor?
A USP tailing factor between 0.9 and 1.2 indicates ideal symmetry, while values above 1.5 suggest significant tailing that needs correction.
Can impurities or tubing affect tailing?
Yes. Contaminants or wide-bore tubing can increase dead volume, broadening peaks and producing tailing artifacts.

